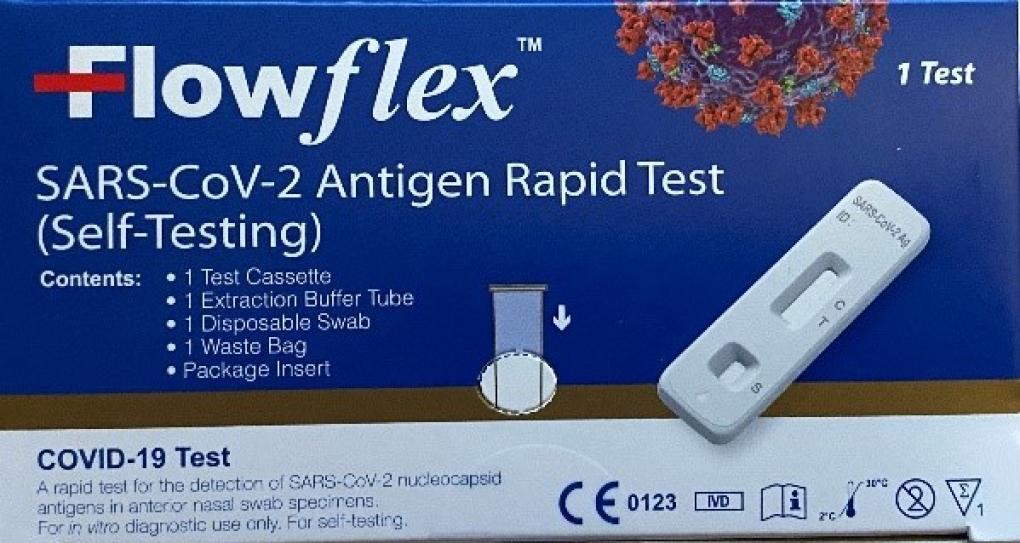
SAN DIEGO, CA. - ACON Laboratories, Inc. announced that they would be recalling their “FlowflexSARS-CoV-2 Antigen Rapid Test (Self-Testing)” from American markets, according to the FDA.
The announcement comes after the discovery of an unauthorized, adulterated and misbranded counterfeit product having the trade name “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) distributing around the U.S.
The Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) is only authorized for sale in Europe.
Those who have received the “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)” with the blue box in the U.S. market, should stop using this product and dispose of it.
The FDA said the "COVID-19 Antigen tests in the U.S. market that lacked FDA approval, clearance, or authorization can pose significant risk since they may lead to inaccurate test results, including false negative or false positive test results. COVID-19 Antigen tests in Europe without the CE mark can pose significant risk since they may lead to inaccurate test results, including false negative or false positive test results."
Any issues or reactions experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Click here to read the entire recall.